
126-33-0
- Product Name:Sulfolane
- Molecular Formula:C4H8O2S
- Purity:99%
- Molecular Weight:120.172
Product Details;
CasNo: 126-33-0
Molecular Formula: C4H8O2S
Appearance: colourless crystals
Chinese Manufacturer Supply Sulfolane, Buy 126-33-0 with Cheapest Price
Sulfolane is an excellent solvent due to its high thermal stability and stability to acid and alkali.
It can be used to extract aromatic hydrocarbons from aliphatic hydrocarbons and to remove acid gases from gas mixtures. It can be used in spinning, film making, etc.
|
Product Name |
Sulfolane |
|
|
CAS |
126-33-0 |
|
|
MF |
C4H8O2S |
|
|
ITEM |
SPECIFCATION |
RESULT |
|
Appearance at 30℃ |
Colorless or canary lucid liquid |
Qualified |
|
Purity, (Dry basis) % |
≥99.5 |
99.72 |
|
Density (30℃)kg/m3 |
1260-1270 |
1263 |
|
5% Distil off temperature ℃ |
≥282 |
285.3 |
|
95% Distil off temperature ℃ |
≤288 |
285.8 |
|
Thermal stability mgSo2/kg |
≤16 |
9.81 |
|
Moisture % m/m |
≤0.1 |
0.022 |
|
Sulfur content % m/m |
26.0-27.0 |
26.52 |
|
2-Sulfolene % m/m |
≤0.2 |
0.017 |
|
Isopropyl sulfolane aether % m/m |
≤0.2 |
0 |
|
Ash content % m/m |
≤0.1 |
0.0042 |
| Packaging | 1kg-15kg packing | 2 PE bag inside + 1 foil bag outside in carton | ||
| 25kg-50kg packing | 2 PE bag inside + 1 foil bag outside in drum | |||
| Other packing | Customized packing | |||
| Drum Size | D38cm*H60cm or customized, 25 kg/ drum | |||
| Shipping | 1-50kg | International Express | Fast and convenient | Door to Door |
| 50-500kg | Air Transportation | Fast and cheap | To Air Port | |
| Above 500kg | Sea Transportation | Cheap and convenient | To Port | |
Sulfolane(Cas 126-33-0) Usage
|
Description |
Sulfolane is a valuable solvent with diverse applications in industries such as refining, chemical synthesis, and membrane fabrication. Its unique properties, including high solubility, stability, and compatibility, make it an essential component in various industrial processes, offering efficient and environmentally friendly solutions for solvent-based applications. |
| Uses |
Refining Processes: Sulfolane is commonly utilized in the refining of natural gas and petroleum, where it serves as a solvent for extraction and purification processes, helping separate valuable components from raw materials. |
InChI:InChI=1/C4H8O2S/c5-7(6)3-1-2-4-7/h1-4H2
126-33-0 Relevant articles
Physicochemical properties of sulfolane
Mario Della Monica, Liliana Jannelli, and Ugo Lamanna
, J. Phys. Chem. 1968, 72, 3, 1068–1071
… in sulfolane was investigated in the molality range 0-1. Data supply evidence that sulfolane … Negative conclusions are drawn concerning the use of sulfolane as a reliable solvent in …
Oxidation of Sulfides with Hydrogen Peroxide to Sulfoxides and Sulfones
Sharipov
, p. 108 - 113 (2003)
Oxidation of sulfides with hydrogen pero...
A Simple Metal Free Oxidation of Sulfide Compounds
Wagh, Ravindra B.,Nagarkar, Jayashree M.
, p. 181 - 187 (2017)
Abstract: This work reports simple, effi...
126-33-0 Process route
-
- 110-01-0
thiophene

-
- 126-33-0,208252-54-4
sulfolane
| Conditions | Yield |
|---|---|
|
With silica gel; magnesium monoperoxyphthalate hexahydrate; In dichloromethane; for 0.833333h; Heating;
|
100% |
|
With sodium tungstate (VI) dihydrate; dihydrogen peroxide; In methanol; water; at 40 ℃;
|
100% |
|
With selenium(IV) oxide; dihydrogen peroxide; In water; ethyl acetate; chemoselective reaction;
|
99% |
|
With oxone; diethylamine; In water; acetonitrile; at 20 ℃; for 0.0833333h;
|
98% |
|
With Oxone; kaolin; In dichloromethane; for 1h; Ambient temperature;
|
97% |
|
With methyltri-n-octylammonium dihydrogenophosphate; sodium tungstate (VI) dihydrate; dihydrogen peroxide; In water; at 20 - 60 ℃; for 2h;
|
97% |
|
With 3-butyl-1-methyl-1H-imidazolium perrhenate; dihydrogen peroxide; 1-butyl-3-methylimidazolium Tetrafluoroborate; In water; at 60 ℃; for 1h; Schlenk technique; Inert atmosphere; Green chemistry;
|
97% |
|
With potassium sulfate; potassium hydrogensulfate; potassium peroxomonosulfate; wet-montmorillonite; In dichloromethane; for 1h; Ambient temperature;
|
96% |
|
With sodium hypochlorite; isocyanuric acid; In water; toluene; at 20 ℃; for 1h;
|
96% |
|
With sodium hypochlorite; water; isocyanuric acid; In toluene; at 20 ℃; for 1h; Inert atmosphere;
|
96% |
|
With potassium permanganate; Rexyn 101 H ion exchange resin; In dichloromethane; for 4h; Heating;
|
95% |
|
With (pyridinium)H3PMo11VO40; dihydrogen peroxide; In water; acetonitrile; at 40 ℃; for 2.5h;
|
94% |
|
With potassium permanganate; In acetonitrile; at 20 ℃; for 4h;
|
93% |
|
With manganese(IV) oxide; potassium permanganate; at 20 ℃; for 0.416667h; ultrasonic irradiation;
|
93% |
|
With potassium permanganate; manganese(II) sulfate; at 20 ℃; for 0.25h;
|
93% |
|
With 4 A molecular sieve; 4-methylmorpholine N-oxide; tetrapropylammonium perruthennate; In acetonitrile; at 40 ℃; for 2h;
|
92% |
|
With N,N'-dibenzyl-N,N,N',N'-tetramethylethylenediammonium bis(permanganate); acetic acid; In acetonitrile; at 20 ℃; for 0.0333333h;
|
92% |
|
With 1,3,5-trichloro-2,4,6-triazine; dihydrogen peroxide; In water; acetonitrile; at 20 ℃; for 0.25h; chemoselective reaction;
|
92% |
|
With dihydrogen peroxide; titanium tetrachloride; In acetonitrile; at 25 ℃; for 0.0333333h;
|
92% |
|
With C2MoO9(2-)*H2O*2C19H42N(1+); dihydrogen peroxide; In water; at 20 ℃; for 0.5h; chemoselective reaction;
|
92% |
|
With Octanoic acid; dihydrogen peroxide; In acetonitrile; at 50 ℃; for 0.416667h; Temperature; Schlenk technique; Green chemistry;
|
92% |
|
With 2,2,2-Trifluoroacetophenone; dihydrogen peroxide; acetonitrile; In tert-butyl alcohol; at 20 ℃; for 3h; pH=11; Green chemistry;
|
92% |
|
With tetra-n-butylammonium hydrogen monopersulfate; In water; at 25 ℃; for 3h;
|
91% |
|
With 1,3,5-trichloro-2,4,6-triazine; dihydrogen peroxide; In tetrahydrofuran; at 20 ℃; for 0.916667h;
|
90% |
|
With dihydrogen peroxide; at 20 ℃; for 0.416667h; chemoselective reaction; Green chemistry;
|
90% |
|
With dihydrogen peroxide; In ethanol; water; at 50 ℃; for 0.416667h; chemoselective reaction; Green chemistry;
|
90% |
|
With urea hydrogen peroxide adduct; tetrabutylammonium phosphomolybdate; at 4 ℃; for 48h;
|
89% |
|
With potassium permanganate; N-benzyl-N,N,N-triethylammonium chloride; In dichloromethane; water; for 12h; Ambient temperature;
|
88% |
|
With urea-hydrogen peroxide; at 85 ℃; for 1h;
|
88% |
|
With potassium permanganate supported on montmorillonite K10; In dichloromethane; at 20 ℃; for 4h;
|
87% |
|
With dihydrogen peroxide; boric acid; at 20 ℃; for 3h; chemoselective reaction; neat (no solvent);
|
87% |
|
With anthracene; oxygen; acetic acid; In isopropyl alcohol; at 75 ℃; for 3h; Irradiation;
|
86% |
|
With aminosulfonic acid; dihydrogen peroxide; In neat (no solvent); at 20 ℃; for 2h; chemoselective reaction; Green chemistry;
|
85% |
|
With 4-methyl-morpholine; osmium(VIII) oxide; In water; acetone; Ambient temperature;
|
84% |
|
With dihydrogen peroxide; In ethanol; water; for 1h; Reflux;
|
81% |
|
With sodium periodate; silica gel; In dichloromethane; for 0.0166667h; Irradiation;
|
72% |
|
With hydrogenchloride; iodosylbenzene; for 0.0833333h;
|
44% |
|
With potassium permanganate; water;
|
|
|
With dihydrogen peroxide;
|
|
|
With dihydrogen peroxide; acetic acid;
|
|
|
With n-heptane; nitric acid;
|
|
|
With copper(II) permanganate; In hexane; for 1h; Ambient temperature;
|
95 % Chromat. |
|
With tert.-butylhydroperoxide; Ce(IV)EPBPSS3; In acetonitrile; at 70 ℃; for 24h;
|
|
|
With [bis(acetoxy)iodo]benzene; oxirane-based polymer-micelle incarcerated ruthenium; In water; acetone; at 20 ℃; for 2h;
|
100 % Chromat. |
|
With phosphomolybdic acid hydrate; oxygen; Cesium dodecyl sulfate; poly(vinylpyrrolidone); In toluene; at 110 ℃; for 16h; under 1520 Torr;
|
|
|
With titania nanoparticles supported on silica; dihydrogen peroxide; In water; acetonitrile; at 40 ℃; for 0.5h;
|
|
|
With 3-chloro-benzenecarboperoxoic acid; In chloroform-d1;
|
|
|
With tetrabutylammonium polychromiumphosphotungstate trihydrate; dihydrogen peroxide; In water; acetonitrile; at 25 ℃; for 0.166667h; chemoselective reaction; Green chemistry;
|
|
|
With water; dihydrogen peroxide; molybdenum(VI) oxide; at 20 ℃; for 18h; Catalytic behavior; Ionic liquid;
|
|
|
With dihydrogen peroxide; In water; acetonitrile; at 50 ℃; chemoselective reaction;
|
|
|
With potassium permanganate; In o-xylene; at 20 ℃; for 3h; under 760.051 Torr; Green chemistry;
|
89 %Chromat. |
|
With bis(N-tert-butylsalicylaldiminato)zinc(II); dihydrogen peroxide; In water; at 50 ℃; for 3.15h; Green chemistry;
|
|
|
With bis(N-isopropylsalicylaldiminato)oxovanadium(IV); dihydrogen peroxide; In neat (no solvent); at 45 ℃; for 1.08333h; Green chemistry;
|
|
|
With C30H22Cl4N4O2Pd2; dihydrogen peroxide; In acetonitrile; at 50 ℃; for 4.15h;
|
|
|
With 4C16H36N(1+)*PW11CrO39(4-)*3H2O; dihydrogen peroxide; In water; at 25 ℃; for 0.166667h; Green chemistry;
|
|
|
With water; N-fluorobis(benzenesulfon)imide; at 20 ℃; for 24h; chemoselective reaction;
|
114 mg |
|
With oxygen; In water; at 50 ℃; for 2h;
|
96 %Chromat. |
|
With molybdophosphoric acid hydrate; dihydrogen peroxide; In ethanol; at 25 ℃; for 0.05h; Reagent/catalyst;
|
-
- 77-79-2
3-Sulfolene

-

- 126-33-0,208252-54-4
sulfolane
| Conditions | Yield |
|---|---|
|
With triethylsilane; 1% Pd on activated carbon; In water; at 45 ℃; for 20h; chemoselective reaction; Green chemistry;
|
100% |
|
With ethanol; platinum; Hydrogenation;
|
|
|
With water; palladium; Hydrogenation;
|
|
|
With ethanol; palladium; Hydrogenation;
|
|
|
With palladium on activated charcoal; ethanol; Hydrogenation;
|
|
|
With ethanol; nickel; Hydrogenation;
|
|
|
With hydrogen; Raney nickel; In water; at 35 ℃; for 1.3h; under 7500.75 Torr; Product distribution / selectivity;
|
|
|
With (bis-1,2-diphenylphosphinoethane)Co(CH2SiMe3)2; hydrogen; In toluene; at -196.15 - 25 ℃; for 7h; under 3040.2 Torr; Reagent/catalyst; Sealed tube;
|
126-33-0 Upstream products
-
110-01-0

thiophene
-
77-79-2

3-Sulfolene
-
17200-23-6
3-Isopropoxythiolane 1,3-dioxide
-
1600-44-8
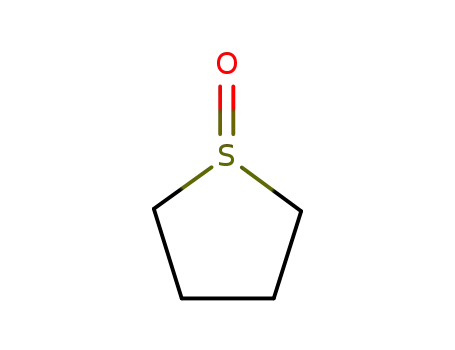
1-oxothiolane
126-33-0 Downstream products
-
6628-06-4
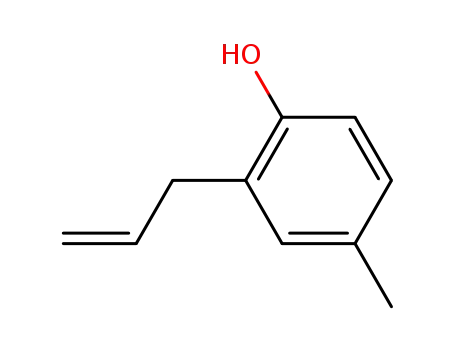
2-allyl-4-methylphenol
-
29866-60-2
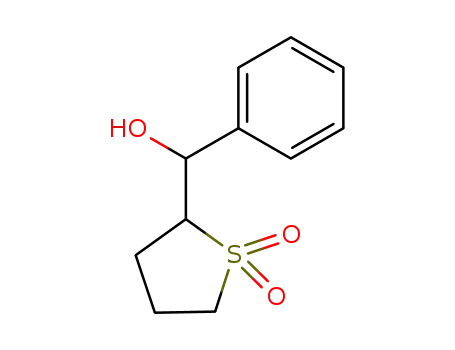
(1,1-dioxo-tetrahydro-1λ6-thiophen-2-yl)-phenyl-methanol
-
53292-12-9
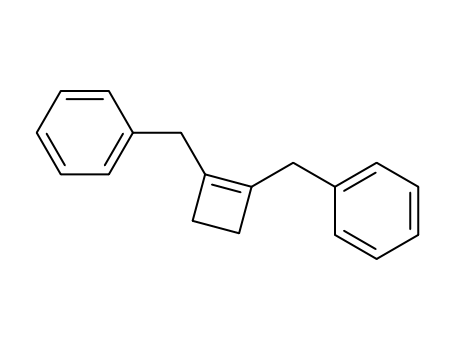
C18H18
-
1501-58-2
1,2-dimethyl-cyclobutene








